In-depth Exploration of DNA Mismatch Repair System
Issuing time 2025-06-04 18:31:39
Discovery of DNA Mismatch Repair System
The mismatch repair system (MMR) was originally discovered in Escherichia coli. The first studies showed that mismatches in DNA molecules induce a repair reaction in the E. coli cell [1].
The performance of polymerases that run DNA synthesis at replication forks is not error-free. The frequency of errors committed by eukaryotic DNA polymerases is estimated at approximately one mistake for every 105 nucleotides, which means that 100,000 errors occur during each cellular S phase. The first line of defense against such a high mutation frequency is the proofreading activity of the polymerase enzymes. Although DNA polymerases insure such lectoring activity by their own domains, some introduced mutations can still slip through unseen and need to be corrected through the second line of defense—the expression of MMR-related genes. MMR is the cellular postreplication process that preserves DNA homeostasis and as such is an evolutionary guarantee of genomic stability [2].
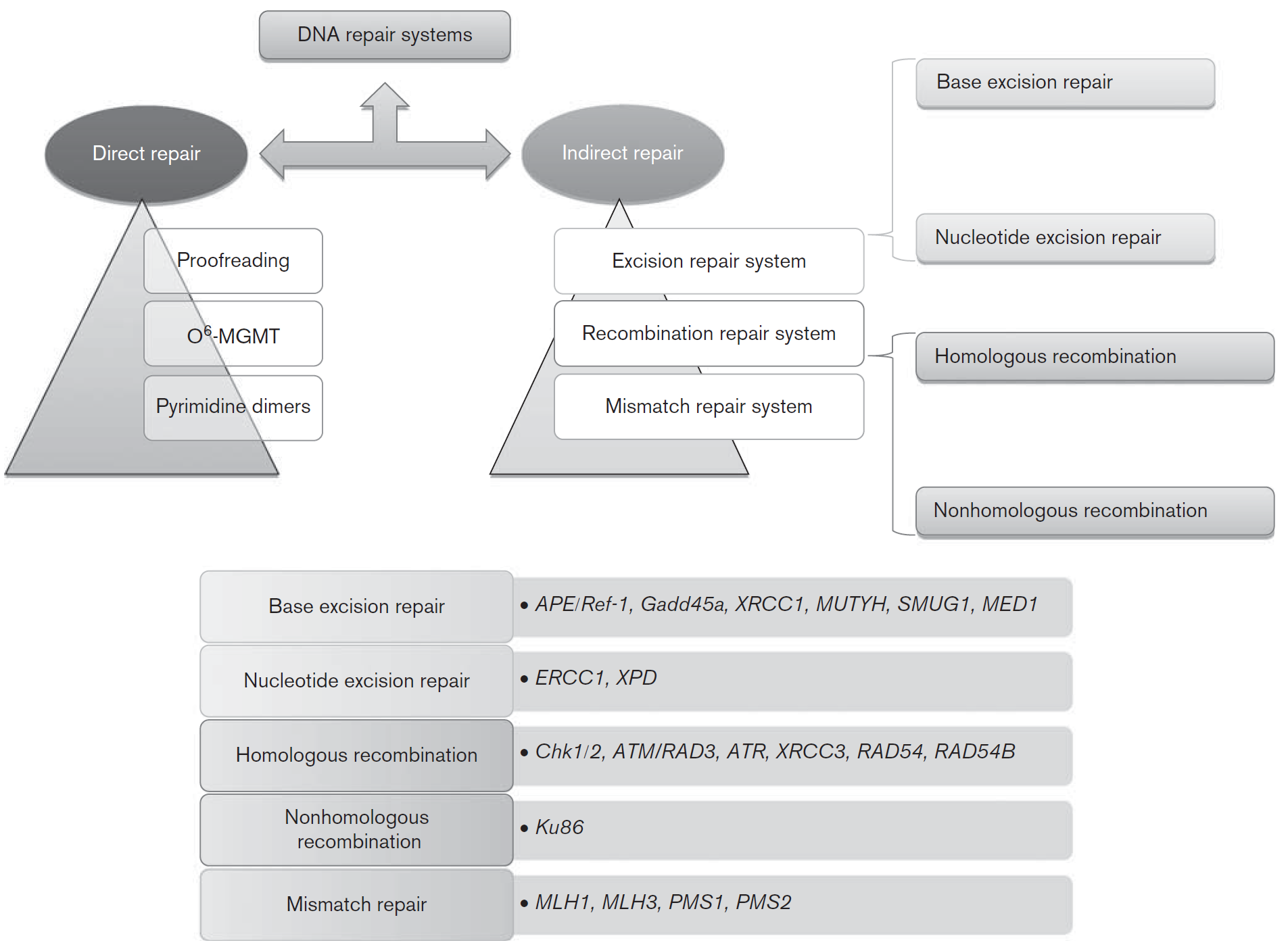
Fig. 1, DNA repair pathways and genes of each pathway involved in colorectal carcinogenesis [3]. MGMT, O6-methylguanine DNA methyltransferase.
Mechanisms of MMR System
Comparative studies on model organisms such as bacteria and Saccharomyces cerevisiae showed that elementary MMR mechanisms and proteins are highly conserved in almost all organisms ranging from bacteria to humans. In order to mediate DNA repair, versatile proteins collectively called MSH and MLH/PMS have evolved in eukaryotes including mammals and humans. All of them fulfill their functions as heterodimers. Their names reflect the homology to the E. coli system, which is why name MSH is short from MutS Homolog, while MLH is derived from MutL Homolog of E. coli [4].
MMR machinery in humans has 8 genes that code for its components. The homologs of E. coli MutS genes in humans are hMSH2, hMSH3, hMSH5, and hMSH6, while MutL homologs are hMLH1, hPMS1 (hMLH2), hMLH3, hPMS2 (hMLH4) [5, 6]. The main job of the DNA mismatch repair system is to correct spontaneous base–base mispairs and small insertions–deletion loops (indels) that are mainly generated during DNA replication. MMR mechanisms involve the following steps: lesion recognition, repair initiation, lesion excision, and DNA resynthesis [7]. Evidence shows that mismatch repair prefers actively transcribed genes [8].
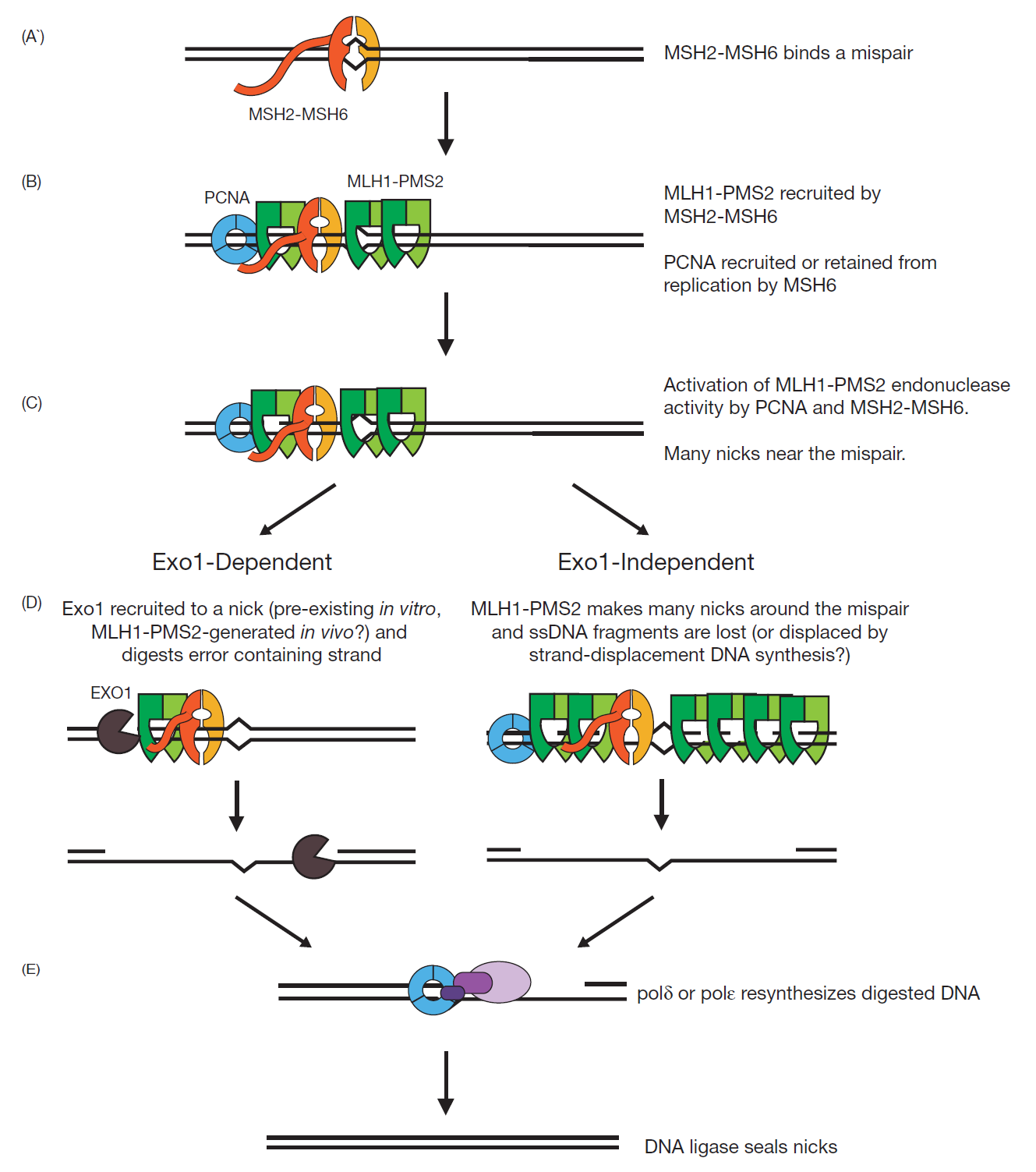
Fig 2, Models for the mechanisms of Exo1-dependent and Exo1-independent MMR reactions [9].
Base–base mispairs are primarily recognized by MSH2-MSH6. The MSH2-MSH6 mispair binding proteins have two composite ATP binding/hydrolysis sites. Binding of ATP, hydrolysis of ATP, and release of ADP at these sites modulates the interaction between these proteins and DNA as well with downstream MMR factors. MLH1-PMS2 is the major MutL-related protein complex that functions in MMR. Mispair bound MSH2-MSH6 recruit and load MLH1-PMS2 onto DNA. There is evidence that either multiple molecules of MLH1-PMS2 are recruited per molecule of MSH2-MSH6 or that MLH1-PMS2 has a longer residence time on DNA than MSH2-MSH6. Moreover, structural and single molecule studies suggest that MLH1-PMS2 also form a clamp on the DNA. MLH1-PMS2 is an endonuclease that makes single strand breaks in DNA, and this activity is essential for MMR.
Its endonuclease activity, which can be observed under a number of different reaction conditions, is highly activated in the presence of MSH2-MSH6, the proliferating clamp nuclear antigen (PCNA), replication factor C (RFC) and ATP on mispaired base containing DNA substrates that also contain a single strand break. Under these reaction conditions, MLH1-PMS2 makes single strand breaks (called nicks) only in the DNA strand that contains the pre-existing nick. EXO1 is a 5’ to 3’ dsDNA exonuclease that can also cleave branched DNA molecules. Considerable data support the idea that EXO1 functions in the mispair excision step of MMR under some conditions and that the interaction between EXO1 and MSH2 increases the extent of excision by EXO1 in response to a mispair. Genetic studies show that loss of EXO1 only causes weak MMR defects, suggesting that other mechanisms for DNA strand excision during MMR must exist. A combination of in vitro MMR reconstitution studies, genetic studies and studies of the MLH1-PMS2 endonuclease established a role for DNA Pol δ, DNA Pol ε, replication protein A (RPA), PCNA and RFC in MMR [9].
Microsatellite Instability and Cancer
Repetitive DNA are immanent and innate sequence elements dispersed in our genome that account for ∼3% of it. Such short repeated DNA sequences are by their nature polymorphic, and are usually known as microsatellites (MS), but can also be referred to as simple sequence repeats (SSRs). They are common across eukaryotic genomes and their length diversity is very high ranging from mononucleotides to hexanucleotides. Microsatellite loci display great variation in population and show multiple alleles that consist of motifs usually repeated between 10 and 60 times [10, 11, 12].
Microsatellites are prone to mutations, especially insertions and deletions, due to DNA polymerase slippage during replication. Detecting mutations in microsatellites, which can be a sign of genomic instability and is used in cancer diagnostics. A high level of microsatellite instability (MSI-H) is the molecular hallmark of inherited or acquired deficiency in MMR (dMMR). Defects in the MMR machinery are mostly acquired as somatic alterations but can also be inherited as germline mutations in the case of Lynch syndrome, with these hereditary mutations resulting in predisposition to a variety of tumour types. Although initially regarded as a mere diagnostic marker of Lynch syndrome, MSI-H/dMMR status is now recognized as a broader cancer phenotype characterized by distinctive clinicopathological, biological, molecular and immunological features with prognostic and therapeutic implications.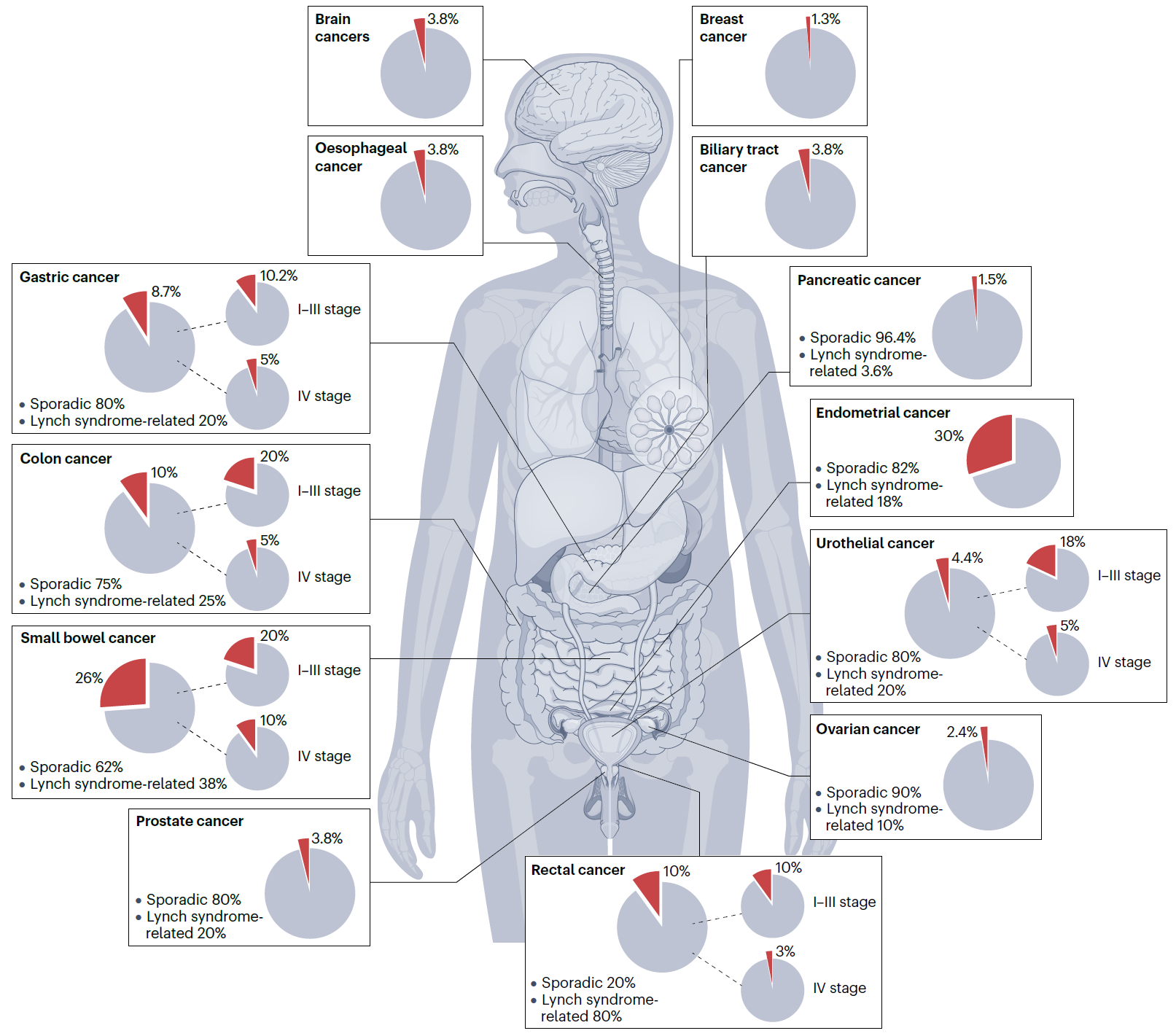
Fig 3, The landscape of the MSI-H/dMMR phenotype across tumour types.
The prevalence of high microsatellite instability/DNA mismatch repair deficient (MSI-H/dMMR) is highly variable across tumour histologies [13, 14, 15, 16, 17, 18, 19, 20, 21, 22], and also varies by stage within individual cancer types. The pie charts depict the prevalence of MSI-H/dMMR tumours for each specific cancer type across all disease stages, as well as stratified early stage (I–III) and advanced-stage (IV) disease, on the basis of the available literature. For cancer types commonly linked to Lynch syndrome, the proportions of sporadic MSI-H/dMMR tumours and Lynch syndrome-related tumours are provided.
Immune Checkpoint Inhibitors approved for MSI-H/dMMR tumors
Immune checkpoint inhibitors (ICIs) are a class of drugs used in cancer immunotherapy that help restore the immune system’s ability to recognize and attack cancer cells. Normally, immune checkpoints are molecules on immune cells (such as T cells) that act as brakes to prevent overactivation, which can cause damage to normal tissues. However, cancer cells often exploit these checkpoints to avoid being attacked by the immune system. ICIs work by blocking these checkpoint proteins—such as PD-1, PD-L1, and CTLA-4—thereby removing the “brakes” and allowing T cells to target and destroy cancer cells more effectively.
In 2017, the US Food and Drug Administration (FDA) approved pembrolizumab (It is a type of ICIs) for any unresectable or metastatic solid tumor with certain genetic anomalies (mismatch repair deficiency or microsatellite instability).This was the first time the FDA approved a cancer drug based on tumor genetics rather than tissue type or tumor site. Subsequently, more immune checkpoint inhibitors (ICIs) received FDA approval [23].
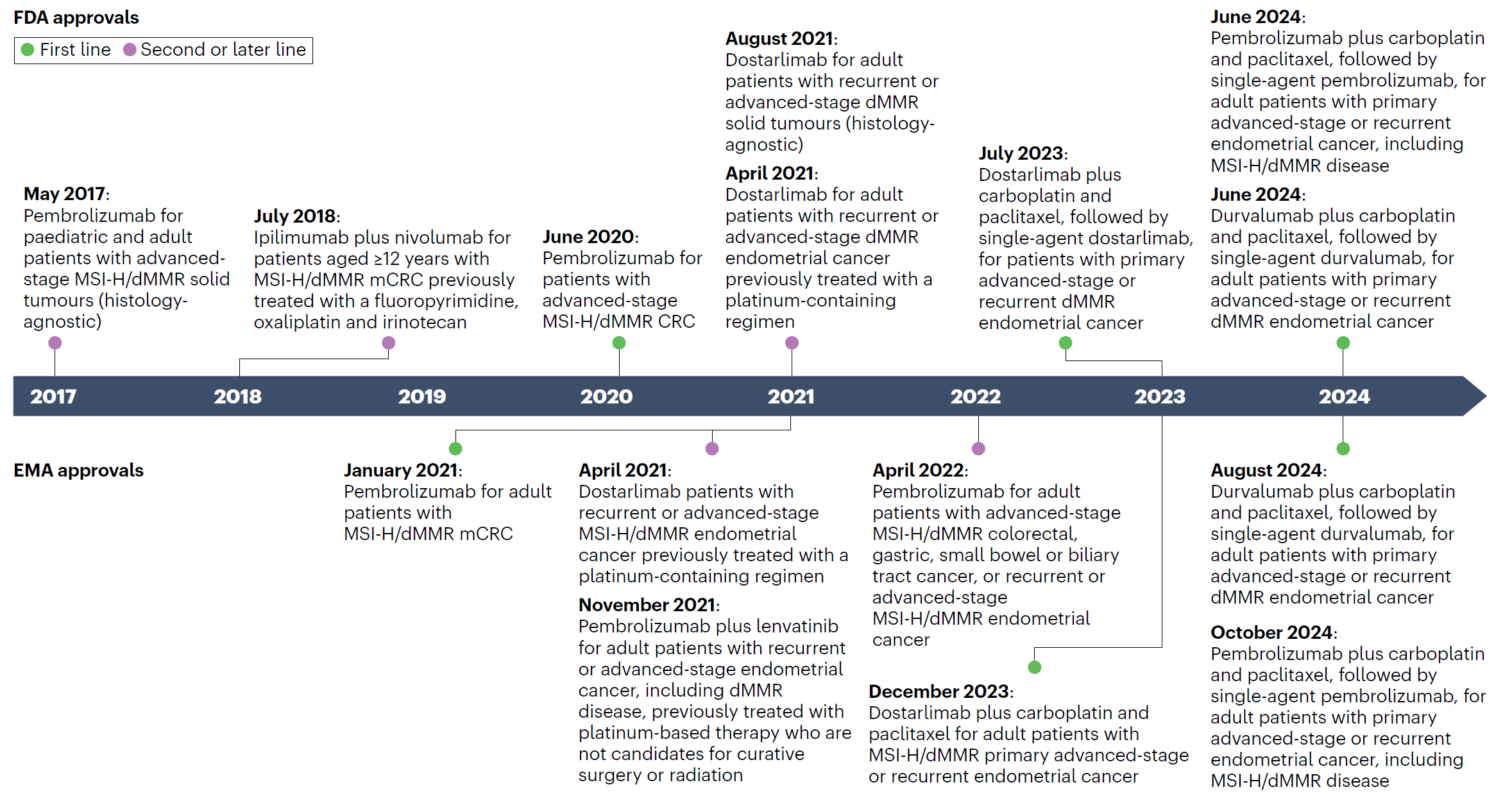
Fig. 4, Timeline of FDA and EMA approvals of ICIs specifically for MSI-H/dMMR cancers.
CRC, colorectal cancer; dMMR, deficient DNA mismatch repair; ICIs, immune-checkpoint inhibitors; mCRC, metastatic colorectal cancer; MSI-H, microsatellite instability-high.
Diagnostic Methods for MMR and MSI
IHC and PCR assays are routinely used to assess MMR and MSI status, respectively, and comprehensive genome profiling (CGP) with assays certified by the Clinical Laboratory Improvement Amendments or other broad NGS panels have more recently been implemented in MSI assessment and might additionally guide genetic counselling. Each modality has advantages and disadvantages (Table 1). Concordance rates among the IHC, PCR and NGS assays range between 90% and 98% [24, 25]. Discrepancies are mostly attributable to heterogenous expression of MMR proteins between tumour cells or different areas in the same specimen, insufficient sample quality or missense MMR mutations [26].
Table 1, Strengths and weaknesses of different MMR/MSI assays
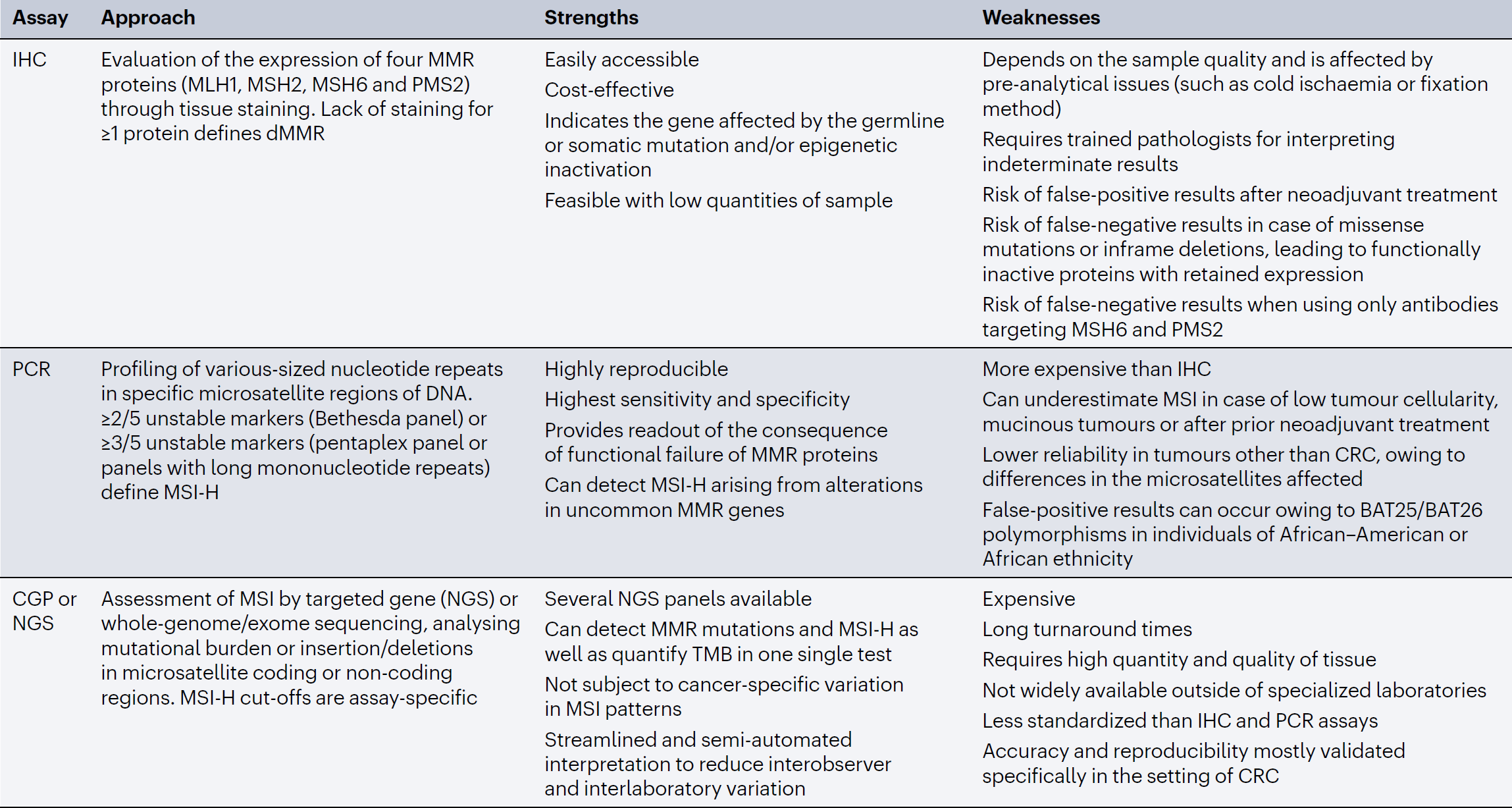
CGP, comprehensive genomic profiling; CRC, colorectal cancer; dMRR, deficient mismatch repair; IHC, immunohistochemistry; MMR, mismatch repair; MSI, microsatellite instability; MSI-H, microsatellite instability-high; NGS, next-generation sequencing; TMB, tumour mutational burden.
IHC is the most widely used MMR/MSI assay in the clinic and is recommended by ESMO as the preferred upfront testing method for all cancers in the Lynch syndrome spectrum [27]. For endometrial cancer, in particular, IHC is the reference method owing to the lower accuracy of PCR testing for MSI compared with CRC, owing to differences in microsatellite instability profiles between these tumour types [28]. IHC staining for MSH2, MSH6, MLH1 and PMS2 is also recommended to limit false-negative results as compared with staining for MSH6 and PMS2 only [29]. In the case of patchy tumour staining and indeterminate results, testing should be repeated on a different tumour block or sample (if available), and if uncertainty remains, use of a different molecular test is recommended [30]. Isolated PMS2 or MSH6 loss can pose a challenge, given that their respective partners can dimerize with other MMR proteins, possibly leading to a dMMR IHC profile but with functionally intact MMR activity (MSS/pMMR). ESMO recommends performing a different molecular test for tumours with an uncertain MMR status on IHC owing to isolated loss of PMS2 or MSH6 staining [27]. Given that isolated MLH1 or MSH2 loss is uncommon, this finding on IHC should also prompt confirmatory molecular testing using a DNA-based MSI assay.
College of American Pathologists (CAP) Guideline for MMR/MSI Testing in Colorectal Cancer
When using immunohistochemistry (IHC) to test MMR, if the result shows the loss of expression of any MMR protein, it is interpreted as defective mismatch repair (dMMR); if all four proteins are expressed, it is interpreted as proficient mismatch repair (pMMR).
In colorectal cancer samples, distinct nuclear staining in lymphocytes, fibroblasts, or normal epithelial cells adjacent to the tumor can be used as internal positive controls.
In the presence of an acceptable internal positive control, any positive reaction of MMR in the nuclei of tumor cells is considered as intact expression (normal), and it is common for intact staining to be somewhat patchy.
In the presence of an acceptable internal positive control, complete loss of nuclear staining of MMR in all tumor cells is considered protein loss.
✨ We, Dartmon, provide the best-in-class MMR IHC antibodies.
✔️ Our MMR IHC antibodies have gotten excellent level from UKNEQAS assessment.
✔️ Moreover, our MMR IHC antibodies have CE and FDA certifications.
Fig. 5, Human tonsil tissue can be used as external positive control. It can be seen from the figure that MMR proteins exhibit intact expression.
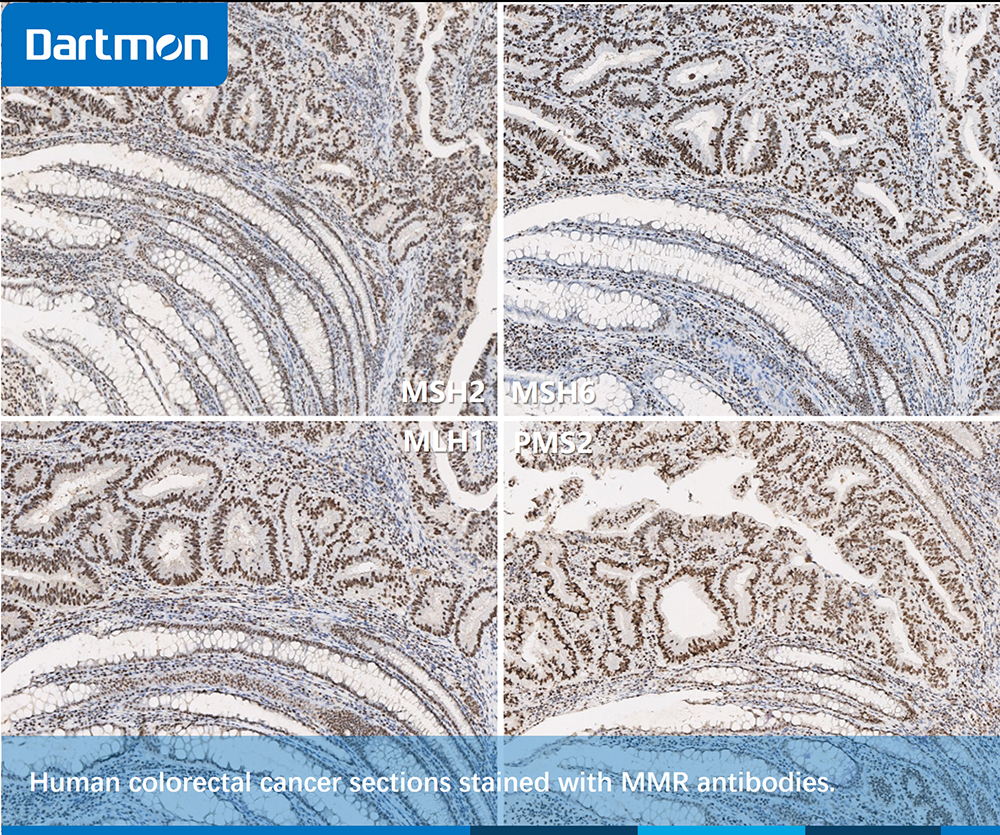
Fig. 6, Human colorectal cancer tissue. It can be seen from the figure that MMR proteins exhibit intact expression.

Fig. 7, Human colorectal cancer tissue. It can be seen from the figure that MSH2 and MSH6 protein exhibit positive expression, while MLH1 and PMS2 proteins are deficient.


